Ph.D., P.E., President
- FMA
- The Fabricator
- FABTECH
- Canadian Metalworking
Categories
- Additive Manufacturing
- Aluminum Welding
- Arc Welding
- Assembly and Joining
- Automation and Robotics
- Bending and Forming
- Consumables
- Cutting and Weld Prep
- Electric Vehicles
- En Español
- Finishing
- Hydroforming
- Laser Cutting
- Laser Welding
- Machining
- Manufacturing Software
- Materials Handling
- Metals/Materials
- Oxyfuel Cutting
- Plasma Cutting
- Power Tools
- Punching and Other Holemaking
- Roll Forming
- Safety
- Sawing
- Shearing
- Shop Management
- Testing and Measuring
- Tube and Pipe Fabrication
- Tube and Pipe Production
- Waterjet Cutting
Industry Directory
Webcasts
Podcasts
FAB 40
Advertise
Subscribe
Account Login
Search
You can machine it, but can you weld it?
Why free-machining steels and welding don’t mix
- By Michael Pfeifer
- October 8, 2012
- Article
- Metals/Materials
Like all manufacturers, metal fabricators often must weigh the desires of product designers with manufacturing costs. The costs include materials, the required fabrication processes, and the time and effort necessary to perform the manufacturing steps. When fabricating assemblies made of carbon and alloy steel, manufacturers may use free-machining steels to make it easier to machine components. However, such steels do contain high levels of sulfur, phosphorus, and lead, which can cause serious welding problems.
This is why welding free-machining steels is usually inadvisable. If one of these steels must be welded, the fabricator can try using low-hydrogen electrodes with a basic slag system. The weld metal's low-hydrogen characteristics will reduce the formation of hydrogen sulfide porosity, and the basic slag system reduces the weld metal's sulfur and phosphorus content. Low welding currents can help minimize dilution with the base metal.
But there is no guarantee that these precautions will eliminate the problem. In fact, sometimes the best that can be achieved is a reduced level of cracking or porosity. The amount of cracking or porosity that is tolerable will depend on the required service conditions and reliability of the weld. Of course, for a weld joint that will be part of a critical, load-bearing structural member, any amount of cracking is unacceptable.
Good communication is critical. In an ideal world, all parties involved in a product's design and manufacturing—from the welders to the manufacturing engineers and designers—should have a basic idea why free-machining steels should not be welded. From here, manufacturing and design engineers should determine the best action. Can welding be avoided by using fasteners? If not, which weldable materials exhibit acceptable machining characteristics, and do these materials meet design requirements?
Metallurgy for Machining
Machining low-carbon steels involves a sequence of metallurgical changes. First, microvoids begin to form, and then grow and coalesce. From this, microcracks form, followed by the ductile fracture and formation of a metal chip.
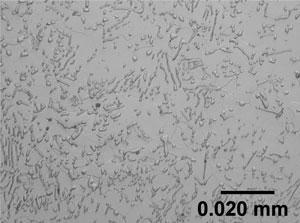
In steels that contain sulfur, the sulfur combines with manganese to form manganese sulfide (MnS) inclusions (see Figure 1). These inclusions enhance the formation of microvoids in the steel during machining, which leads to the formation of broken chips rather than continuous chips. However, steels are typically specified with only 0.050 percent sulfur maximum, because steel weldability decreases with increasing sulfur content. So, the number of MnS inclusions is small.
Free-machining steels, on the other hand, have more than 0.050 percent sulfur. Resulfurized steels usually have sulfur content between 0.08 and 0.13 percent, and some grades have sulfur content as high as 0.35 percent. The additional sulfur results in more MnS inclusions in the steel, enhancing the formation of microvoids during machining.
Phosphorus and lead also may be added to resulfurized low-carbon steel. Standard carbon steels have no more than 0.040 percent phosphorus. In rephosphorized free-machining steels, however, the phosphorus content is 0.04 to 0.12 percent.
Phosphorus increases the strength and hardness of the ferrite phase in the steel. In some operations, this promotes chip breaking rather than the formation of long, stringy chips. Excessive phosphorus, however, impairs machining characteristics, while decreasing as-rolled steel ductility and notch impact toughness.
Lead additions to free-machining steel are generally in the range of 0.15 to 0.35 percent. Lead is present in steel as soft particles that enhance the formation of microvoids during machining.

Figure 1: In steels that contain sulfur, the sulfur combines with manganese to form manganese sulfide (MnS). This enhances the formation of microvoids during machining, which aids chip formation. MnS does have a low melting point, however, which is why excessive amounts of it can cause welding problems.
Why Welding Problems Occur
Free-machining steels with sulfur and phosphorus have relatively poor weldability because cracks can form during the final stages of weld solidification. The cracks arise from the presence of liquid MnS and phosphorous compounds at the weld metal grain boundaries. These materials have a lower melting point than other elements in the steel. As the weld metal cools and solidifies, stresses build across the weld due to shrinkage. However, the low-melting-temperature compounds remain molten. Consequently, the grains tear apart under the shrinkage stresses. High sulfur content also promotes weld metal porosity.
Normal amounts of sulfur and phosphorus do not promote weld metal cracking. Plain carbon steels containing less than 0.30 percent carbon and 0.05 percent sulfur can be welded readily by most methods with little need for special measures. Medium-carbon steel (0.30 to 0.60 percent carbon) can be successfully arc-welded provided suitable precautions are taken.
Occasionally lead may cause weld porosity and embrittlement, but the major concern with this element involves welding fumes. A toxic element, lead melts quickly during welding and can volatize into the welding fumes.
Machinability and Weldability
The best solution usually involves selecting a standard carbon steel alloy with a composition, microstructure, and hardness that balances acceptable machining with ease of welding, while meeting product performance and reliability requirements. Machining problems can be a major source of headaches, which is why free-machining steels look so attractive. But there are ways to achieve acceptable machinability without additional sulfur, phosphorus, or lead.
To understand what aids the machining process, it helps to know some basic metallurgy. The metallurgical phases present in low- and medium-carbon steel typically include ferrite and spheroidized cementite, or ferrite and pearlite. Ferrite is a soft material, and cementite is a hard material (see Figure 2). Pearlite is a composite consisting of plates of ferrite and cementite. The amount of cementite or pearlite in a steel increases as carbon content increases.
A few different heat treatments are used to obtain these different microstructures. Normalizing and full annealing result in ferrite and pearlite. Spheroidization annealing can obtain ferrite and spheroidized cementite.
In a machining center, ferrite can be readily cut and causes little tool wear. Because of its low hardness, however, it does contribute to the formation of a built-up edge (BUE) on the cutting tool. Cementite particles are very hard, and large quantities of them can cause significant cutting tool wear. Pearlite is harder than ferrite and generally causes tool wear as well, with wear increasing as the pearlite plate spacing decreases. However, BUE on the cutting tool is less common when machining pearlite than when machining ferrite. Among normalized and annealed steels, those with lower hardness and smaller amounts of pearlite can be machined at higher speeds without sacrificing cutting tool life.
The machinability of as-rolled or annealed low-carbon steel improves with increasing pearlite content and smaller ferrite grain sizes. This occurs because this microstructure promotes microvoid formation at the interface between pearlite and ferrite. Again, those microvoids promote efficient chipping under the cutting tool.
Maximum machinability of low-carbon steels is achieved at 0.15 to 0.25 percent carbon in the as-rolled or annealed condition. With medium-carbon steels, normalizing or annealing combined with cold drawing gives a slight increase in machinability compared to as-rolled cold-drawn steel.
Cold working increases the hardness of ferrite, which results in shorter chip lengths and less BUE on the cutting tool. However, using cold-worked steel may require a stress-relief heat treatment prior to machining to minimize distortion.
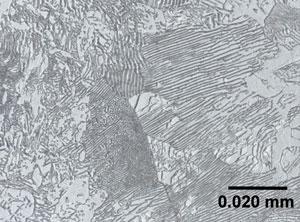
Figure 2: This shows ferrite and pearlite phases. The dark-colored material is cementite (which is part of the pearlite composite) and the light-colored material is ferrite.
A Balancing Act
As with so much in manufacturing, finding the right steel is a balancing act from both a metallurgical and financial perspective. The goal is to find a weldable steel that allows the machining center's cutting tool to break away small chips efficiently. Choosing steels with acceptable machinability, without relying on free-machining grades, may increase material costs. But those increased costs may be easily offset by a reduction in welding problems.
Yes, free-machining steel will have better machinability attributes than standard steel. This is why a trade-off analysis should determine lowest cost while meeting or exceeding requirements in machinability, weldability, and product reliability.
About the Author
Michael Pfeifer
900 Hawthorne Lane
Northbrook, IL 60062
847-528-3467
subscribe now

The Fabricator is North America's leading magazine for the metal forming and fabricating industry. The magazine delivers the news, technical articles, and case histories that enable fabricators to do their jobs more efficiently. The Fabricator has served the industry since 1970.
start your free subscription- Stay connected from anywhere

Easily access valuable industry resources now with full access to the digital edition of The Fabricator.

Easily access valuable industry resources now with full access to the digital edition of The Welder.

Easily access valuable industry resources now with full access to the digital edition of The Tube and Pipe Journal.
- Podcasting
- Podcast:
- The Fabricator Podcast
- Published:
- 04/16/2024
- Running Time:
- 63:29
In this episode of The Fabricator Podcast, Caleb Chamberlain, co-founder and CEO of OSH Cut, discusses his company’s...
- Industry Events
16th Annual Safety Conference
- April 30 - May 1, 2024
- Elgin,
Pipe and Tube Conference
- May 21 - 22, 2024
- Omaha, NE
World-Class Roll Forming Workshop
- June 5 - 6, 2024
- Louisville, KY
Advanced Laser Application Workshop
- June 25 - 27, 2024
- Novi, MI